
Tailoring Clinical Trials Ensures Launch Success, Maximizes Budgets
Enhance Efficiency and Patient Engagement in Clinical Trials with Expert Support.

With increased market demand for the introduction of specialty pharmaceuticals to treat complex, chronic conditions, life sciences companies are pressured to make global clinical trials faster, less expensive and more accurate without compromising quality or integrity. Maximizing efficiency in terms of time and money are key objectives, with AscellaHealth contributing meaningful services and support in the clinical trial process. Our team includes individuals who hold NIH certifications in the management of clinical trial activities, ensuring dedicated expertise and capabilities in this area.
Strategies to Optimize Clinical Trials
Focus on Patient-Centered Engagement, Patient Recruitment and Retention
Maintaining patient engagement and minimizing dropout rates in clinical trials are persistent challenges in terms of wasted time and resources, loss of valuable data and impact on patient well-being. Failure to recruit or retain a sufficient number of participants may lead to trial delays that stall marketing application filing, issues that could result in a potential loss of $8 million per day for drug developers.
To address these challenges, AscellaHealth partners with life sciences manufacturers to assist in ways that ensure compliance and retention of potential participants. Our dedicated patient care teams help participants navigate the clinical trial journey. We help in providing education and support to patients around the importance of protocols or the purpose of the study, building relationships with patients that promote trust and satisfaction throughout the trial. The results improve their experience, resulting in less patient fatigue and clinical trial dropout.
Our teams maintain longstanding relationships with healthcare professionals who specialize in the care and treatment of patients with complex disorders, as well as patient advocacy organizations and disease-specific registries. These relationships allow us to bridge the gaps between patients, healthcare professionals and pharmaceutical companies as they develop protocols and recruit for clinical trials. Through our long-standing history and experience with clinical trials, we assist in all aspects including obtaining necessary consent forms, communicating eligibility requirements, process enrollments and ensure we maintain compliancy with all regulatory requirements.
Data, Analytics and Real-World Evidence
By providing a reliable, consistent source of accurate data, intelligence and real-world evidence (RWE) / real-world data (RWD) to identify eligible patient populations for clinical trials, our teams streamline the process, offering end-to-end solutions for product manufacturers. This approach reduces clinical trial costs by decreasing the need for extensive vendor management, while delivering accurate results in the shortest possible time frame.
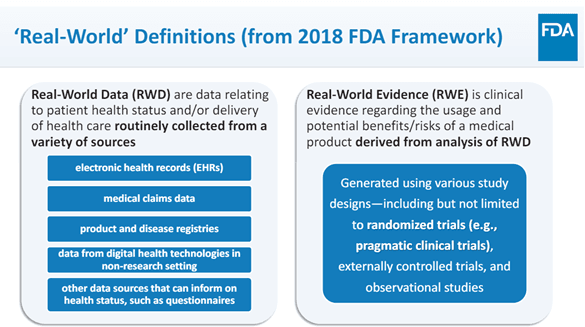 Photo courtesy of: https://publichealth.jhu.edu/sites/default/files/2023-09/rare-disease-day-1-slides-05022023.pdf
Photo courtesy of: https://publichealth.jhu.edu/sites/default/files/2023-09/rare-disease-day-1-slides-05022023.pdf
Appropriate Study Design
Identifying the most appropriate study design is important for generating the best evidence to answer a clinical research question. A well-designed study will clearly identify a range of variables, including the participant population, the intervention or exposure of interest, the outcomes to be investigated and how data will be collected. Robust study designs generate reliable data and facilitate evidence-based decision-making.
Manufacturers worldwide turn to us for assistance with the design and conduct of a clinical trial, including devising the appropriate participant selection criteria and providing information to investigators monitoring the trial.
Expanded Access Programs
Expanded access, also known as compassionate use, is a potential pathway for patients with an immediately life-threatening condition or serious disease who do not meet trial criteria and are deemed ineligible for a clinical trial. These programs enable patients to gain access to an investigational medical product (drug, biologic or medical device) outside of clinical trials when no comparable or satisfactory alternative therapy options are available.
AscellaHealth helps manufacturers to manage all aspects of their Expanded Access Programs (EAPs) until the product becomes approved and our team can transition all patients to the commercially available product.
Partner with a Single-Source Expert
As a trusted, single-source global partner with deep expertise and experience with specialty pharmaceuticals, AscellaHealth improves the quality and integrity of the clinical trial process by providing tech-enabled end-to-end solutions, support and resources that result in faster, more cost-effective and successful trials.
Stay Connected
For more information, please contact businessdevelopment@ascellahealth.com.